Introduction: B-cell lymphoma factor 2 (BCL-2) family, as an important factor regulating apoptosis, is abnormally expressed in most hematological malignancies. Clinical trials of venetoclax(VEN) combined with conventional chemotherapy for pediatric AML are ongoing. Previous research has shown that the combination of HHT and VEN can synergistically promote cell apoptosis. Therefore, a phase I study utilizing HAG combined with VEN, in patients with de novo AML pediatric was undertaken to evaluate the safety and feasibility.
Methods: This ongoing single center Phase I trial combining 11 days of VEN, 10 days of Ara-c plus G-CSF with increasing doses of HHT, namely HAGV. VEN was given as a ramp-up over 1 day, followed by a fixed dose of 240 mg/m 2 PO QD over a total period of 10 days, Ara-c 10 mg/m 2/q12h IVGTT fixed dose over 10 days and G-CSF 5ug/kg IH fixed dose over 10 days. Three dose levels of HHT, 1mg/m 2(dose level 1 group), 2/m 2(dose level 1 group) and 3mg/m 2(dose level 3 group) daily were studied in a 3 × 3 dose-escalation design to define maximum tolerated dose (MTD). The primary objective was to determine safety and tolerability. Adverse events (AE) and dose-limited toxicities (DLT) were monitored throughout treatment. The secondary objective was to evaluate preliminary efficacy. The trial is registered under ChiCTR2200064901.
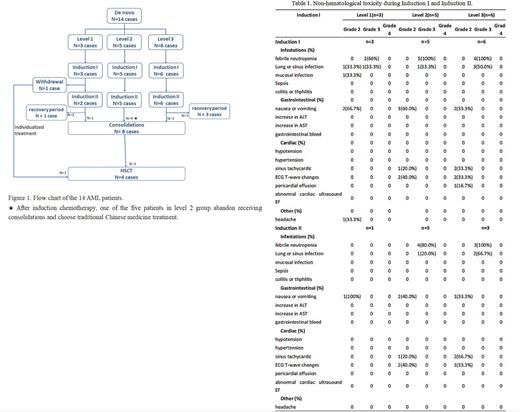
Results: Between October 2022 and June 2023, 14 patients [50% female, median age 100 months (range 39-153)] were enrolled in the dose level 1(n=3)、dose level 2(n=5) and dose level3(n=6) portions of the study (Figure 1). Four (28.6%) had normal cytogenetics. The most common fusion gene was RUNX1::RUNX1T1(n=5, 35.7%), followed by MLL-r (n=2, 14.3%, 1 case with MLL-AF6 and 1 case with MLL-AF10), CBFβ-MYH11(n=1, 7.1%) and NUP98-NSD1(n=1, 7.1%). The most common mutations identified were NRAS (n=4, 28.6%), CEBPA-dm (n=3, 21.5%), KIT (n=3, 21.5%), CEBPA-sm (n=2, 14.3%) and KRAS (n=2, 14.3%). All patients completed one cycle of HAGV as Induction I. One patient in dose level 1 group achieved NR after Induction I and then withdraw from the trail. Eventually, total 13 patients received two cycles of HAGV as Induction II. No DLTs were observed. There were no deaths in the first 30 days from treatment initiation. During Induction I, the most common non-hematological toxicity regardless of grade and attribution included febrile neutropenia(n=13, 92.9%), nausea or vomiting (n=7, 50.0%), lung infection(n=6, 42.9%), ECG T-wave changes (n=4, 28.6%) and sinus tachycardic (n=3, 21.4%) (Table 1). All the 14 patients experienced at least one grade 3 AE, with thrombocytopenia (n=14, 100%), neutropenia (n=14, 100%), and febrile neutropenia (n=13, 92.9%) most common. The median time of neutropenia and platelet recovery time were 22 days(range 14-38)and 16 days(range 10-28) in Induction I, respectively. During Induction II, 4 of the 13 patients are still in the recovery period and has not reached the evaluation point. Among the remaining 9 patients, the most common non-hematological toxicity included febrile neutropenia(n=7, 77.8%), nausea or vomiting (n=4, 44.4%), ECG T-wave changes (n=3, 33.3%), sinus tachycardic (n=3, 33.3%) and lung infection(n=2, 22.2%). The median time of neutropenia and platelet recovery time were 18 days(range 7-34)and 15 days(range 0-29) in Induction II, respectively. Serious AEs of grade 4-5 were not observed during Induction therapies. Remission assessment showed CR in 11/14(78.6%) patients after Induction I and 8/9(88.9%) patients after Induction II.
Conclusions: We have determined that the combination of VEN plus HAG is safety and HHT at doses of 1, 2 and 3mg/m 2 was well tolerated. No DLTs were observed, and the most common Grade 3 non-hematological toxicity were febrile neutropenia.
Disclosures
No relevant conflicts of interest to declare.